nitrogen electron configuration|nitrogen electron configuration long form : Manila Nitrogen Electron Configuration. Wayne Breslyn. 746K subscribers. Join. Subscribed. 541. 120K views 10 years ago. A step-by-step description of how to write the electron . Live streaming of the 2024 U.S. Open Golf Championship at Pinehurst No. 2 (NC), June 13-16. Watch some of the world's best players with Featured Groups coverage, highlights, and more! Pinehurst Resort & C.C. (Course No. 2) • .
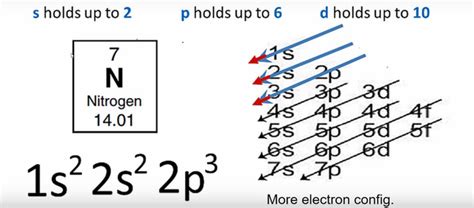
nitrogen electron configuration,Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit .Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can . Find the electron configuration of any element using this tool. Learn the rules, notation, and examples of electron configuration, including nitrogen (1s2 2s2 2p3).
Nitrogen Electron Configuration. Wayne Breslyn. 746K subscribers. Join. Subscribed. 541. 120K views 10 years ago. A step-by-step description of how to write the electron . The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is [He]2s22p3. The atomic number of nitrogen is 7, .If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, a very important element in the biology of plants: 1s 2 2s 2 2p 3 We can clearly see that p orbitals are half-filled as there are three electrons .
Learn how nitrogen acquires its valence electrons and becomes present in most proteins, playing a very important role in certain biochemical applications. The electron . The electron configuration of nitrogen is 1s2 2s2 2p3. You can see the image below. What is the Orbital Diagram for Nitrogen? For drawing the orbital diagram configuration of the nitrogen we have to .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
nitrogen electron configuration long formThe electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and .The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two .The electron configuration of nitrogen is 1s22s22p6. Nitrogen becomes present in most proteins, playing a very important role in certain biochemical applications, such as industrial applications. This element has a great ability to create triple bonds when combined with another nitrogen atom and with other types of elements, where strong bonds .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .

The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 . The full electron configuration for nitrogen is "1s"^ 2"2s"^2"2p"^3. The noble gas shorthand electron configuration is ["He"]"2s"^2"2p"^3". The atomic number of nitrogen is 7. This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electron configuration will .
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. . This diagram represents the correct filling of electrons for the nitrogen atom. (b) This diagramrepresents the . The electron configuration of nitrogen is 1s 2 2s 2 2p 3, helps to express how the electrons are arranged in nitrogen’s atomic orbital.Let us study its electron configuration in detail. Nitrogen, a non-metal is found to be the lightest element of group 15 of periodic table.It is the 4 th highest electronegative element. It is a p-block element.
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, a very important element in the biology of plants: 1s 2 2s 2 2p 3. We can clearly see that p orbitals are half-filled as there are three electrons and three p orbitals. This is because Hund's Rule states that the three electrons in the 2p subshell will fill all the .

Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Nitrogen in the form of ammonium chloride, NH 4 Cl, was known to the alchemists as sal ammonia. It was manufactured in Egypt by heating a mixture of dung, salt and urine. Nitrogen gas itself was obtained in the 1760s by both Henry .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first .
nitrogen electron configuration The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Example 1.6.1 1.6. 1: Nitrogen Atoms. Consider the correct electron configuration of the nitrogen (Z = 7) atom: 1s 2 2s 2 2p 3. The p orbitals are half-filled; there are three electrons and three p orbitals. This is because the three electrons in the 2p subshell will fill all the empty orbitals first before pairing with electrons in them.The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and . To write the orbital diagram for the Nitrogen atom (N) first we need to write the electron configuration for just N. To do that we need to find the number o. Let's find the electron configuration of Nitrogen! A single Nitrogen atom has 7 protons and 7 electrons, but how do we know where Nitrogen puts its electrons. Answer link. For the nitrogen atom, Z=7 And so we distribute 7 electrons: 1s^ (2)2s^ (2)2p^ (3)..the p electrons occupy each of the 2p orbitals, p_x, p_y, p_z singly..this is the most stable electronic configuration.nitrogen electron configuration nitrogen electron configuration long form Electron Configuration: 1s 2 2s 2 2p 3: Valence Electrons: 2, 5: Phase: Gas: . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple .
nitrogen electron configuration|nitrogen electron configuration long form
PH0 · what are the 2s electrons in nitrogen
PH1 · nitrogen electron configuration long form
PH2 · how to draw molecular orbital diagram
PH3 · how many electrons does nitrogen have
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · complete the electron configuration for n
PH8 · Iba pa